As someone who spends a lot of time tuning reverse osmosis (RO) systems for both homes and light commercial applications, I can tell you that pH is one of the most quietly powerful levers you have. People often focus on filter stages, gallons per day, or “how many contaminants this system removes.” Those matter. But the pH of the water going into and through your RO membrane quietly determines how long that membrane lasts, how well it rejects contaminants, how often you have to clean it, and even how your drinking water tastes.
This article will walk you through what pH really means in an RO context, why the pH window is narrower in practice than the “paper specs,” how low and high pH damage membranes in different ways, and what practical steps you can take to keep pH in the safe zone while still supporting healthy, enjoyable hydration at home.
pH 101: What pH Really Tells You
pH is a measure of how acidic or alkaline water is on a scale from 0 to 14. A pH of 7 is neutral. Numbers below 7 are acidic, and numbers above 7 are alkaline. Multiple sources, including Fliers Quality Water and various RO manufacturers, emphasize this basic scale because it is the foundation for understanding almost everything about water chemistry.
In natural and tap waters, pH is largely controlled by dissolved minerals and carbonate species. Calcium and magnesium salts, especially their bicarbonate forms, tend to make water slightly alkaline and act as buffers that prevent pH from swinging too far. When those minerals are removed, as in reverse osmosis, the water loses much of its buffering capacity and becomes much more sensitive to small amounts of acid or base.
In RO systems, pH is not just a comfort or taste parameter. It directly influences membrane chemistry, scaling and fouling, how efficiently ions are rejected, and how aggressively you need to operate and maintain your system. That is why technical guidance from companies like AXEON and DuPont treats pH as a primary design and control variable, not a side note.

How RO Membranes Work – And Where pH Fits In
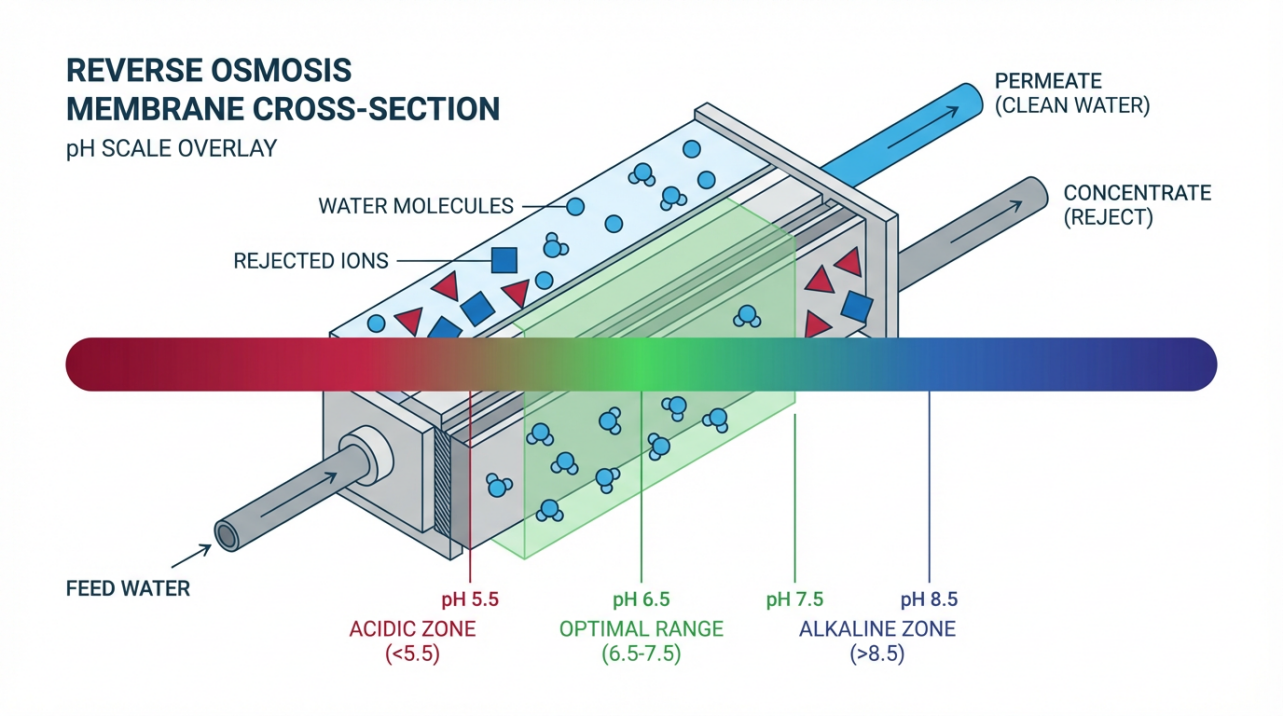
Reverse osmosis forces water under pressure through a semi‑permeable membrane. The membrane behaves like a molecular sieve: water molecules pass, while most dissolved salts, organics, and particles are held back and flushed away in a concentrate (or brine) stream. Resources from Puretec Water and Waterdrop describe this as producing two streams: permeate, which is your purified water, and concentrate, which carries the rejected contaminants.
Key performance metrics for an RO membrane include permeate flux (how much water passes through per area), salt rejection (how well dissolved ions are held back), and recovery (what fraction of feed water is converted into permeate). These metrics all respond to pressure, temperature, and feed-water chemistry. Among those chemistry factors, pH is one of the most influential because it affects:
Membrane surface charge and how strongly the membrane repels different ions, as highlighted by research on pH effects in RO membranes published in ScienceDirect.
The solubility of scale-forming minerals like calcium carbonate and silica, which drive deposits on the membrane surface.
The corrosiveness of the water toward system components, including the membrane itself and any metal plumbing downstream.
Whether oxidants, disinfectants, and cleaners are in a form that is safe for the membrane or likely to attack it, a point emphasized in guidance from Sensorex and DuPont.
Because of these interactions, pH is not something you can ignore and hope the membrane “just handles it.” The membrane is engineered with specific pH tolerances, and performance drops noticeably when you drift outside the optimal band.
The Ideal pH Window for RO Membrane Health
On paper, many modern RO membranes are rated to survive a wide pH range, often somewhere around pH 2 to 11, especially during short-term cleaning. AXEON notes that membranes can technically tolerate this chemistry, but their own performance data show a much narrower sweet spot for everyday operation.
Several sources converge around similar guidance. BestMembrane notes that RO membranes generally perform best between roughly pH 6 and 8. AquaComponents describes an optimal operating window of about pH 5.5 to 8.5, warning that operation beyond this band drives degradation, reduced filtration efficiency, and more aggressive scaling or irreversible fouling. AXEON’s pH and bicarbonate management guidance tightens that practical sweet spot even further, showing flux rates highest around pH 6.5 to 7.5 and reporting that flux can drop by roughly 20 percent when you run outside this range, even though the membrane is technically still “within spec.”
To put the different pH bands in perspective, consider the following summary.
pH range |
Typical effect on RO membranes and system behavior |
Below about 5.0 |
Water becomes more corrosive; AquaComponents notes polymer deterioration and declining performance. BestMembrane indicates membranes are prone to hydrolysis in this zone. |
Around 5.5–6.5 |
Lower edge of acceptable operation. pH control is critical to avoid corrosion and to maintain good ion rejection. |
Around 6.5–7.5 |
Practical sweet spot cited by AXEON, where flux and salt rejection are near peak and chemical damage is minimized. |
Around 7.5–8.5 |
Still considered acceptable for many membranes. Scaling risk rises as pH, alkalinity, and temperature increase. |
Above about 9.0 |
AquaComponents warns of higher scaling from calcium, magnesium, and silica; water becomes more prone to mineral precipitation. |
Above about 10.5 |
AquaComponents describes conditions in this range as likely to trigger irreversible fouling and permanent membrane damage. |
The key idea is this. The “survival range” on a data sheet is wide, but the “happy range” where you get long membrane life, strong rejection, and manageable cleaning frequency is much narrower and centered near neutral pH.
What Happens When pH Is Too Low
Running an RO membrane at consistently low pH might feel safe because low pH can reduce carbonate scaling. However, the research and field experience reflected in the sources here show that you pay a price for operating too acidic.
BestMembrane points out that at pH values below about 6, membranes are prone to hydrolysis. Hydrolysis is a chemical breakdown of the membrane material, especially common in some thin-film membranes when exposed to acidic conditions for prolonged periods. AquaComponents notes that below roughly pH 5, water becomes more corrosive, which can damage polymer layers and adhesives, as well as any susceptible metallic components. Durpro echoes this by warning that extreme pH values compromise both permeate quality and infrastructure, with low pH water more aggressively leaching metals into downstream pipes and tanks.
Low pH can also alter ion rejection in complex ways. Research summarized on ScienceDirect shows that as feed pH decreases, the surface charge of certain RO membranes changes, which affects how cations and anions are excluded. In some systems, chloride rejection falls while sodium rejection rises as pH drops below a certain threshold. That kind of asymmetric behavior can reduce overall desalination performance and lead to unexpected ion balances in the permeate.
None of this means that you can never use acidic chemistry in an RO system. In fact, targeted use of low-pH cleaners is one of the standard ways to remove mineral scale. Crystal Quest, for example, describes a low-pH membrane cleaner mixed at about 1 pound per 10 gallons of water and maintained around pH 2 to dissolve deposits of calcium, magnesium, iron, and other mineral scales. The crucial difference is that this cleaning is temporary, done under controlled conditions, and followed by thorough rinsing, rather than being the continuous operating state of the system.
For day-to-day operation, hovering near neutral rather than mildly acidic gives you a much better balance of scale control, membrane stability, and consistent water quality.

What Happens When pH Is Too High
If low pH stresses the membrane material, high pH mainly attacks your system through scaling. AquaComponents warns that above a pH of about 9, scale formation from calcium, magnesium, and silica increases, which reduces water flow and salt rejection. In severe cases, especially when pH exceeds about 10.5, they note that irreversible fouling and permanent membrane damage are likely.
AXEON’s technical guidance on pH and bicarbonate management explains why. In alkaline conditions, carbonate chemistry shifts. Dissolved carbon transitions from carbon dioxide and carbonic acid at lower pH to bicarbonate and carbonate ions as pH rises. Durpro points out that alkaline conditions favor carbonate ion formation and that these carbonate ions readily combine with calcium to form calcium carbonate deposits. These deposits appear as hard scale on the membrane surface, restricting water passage and forcing you to run at higher pressures for the same permeate flow.
Scaling is especially problematic in systems that are both warm and high recovery. AXEON notes that for each increase of about 10 degrees in temperature, calcium carbonate solubility drops significantly, which means that a warm system operating at high recovery and high pH can cross into heavy scaling very quickly. Their use of the Langelier Saturation Index (LSI) shows that as conditions move from an LSI of roughly minus two to plus two, the risk shifts from non-scaling through slight scaling to heavy scaling.
High pH also changes the electrical behavior of the membrane. Durpro observes that elevated hydroxide ion concentrations can raise permeate conductivity and alter mineral content, even when other quality indicators look acceptable. This can create the illusion of adequate purification if you look only at bulk conductivity, especially when significant carbonate or bicarbonate is present.
Again, there is a controlled role for high-pH chemistry. Crystal Quest describes high-pH cleaners used for organic fouling, maintaining cleaning solutions around pH 12 to break up biofilm, algae, and slime on membranes. These treatments are designed for limited time windows, with solution temperatures typically kept at or below about 86°F and thorough flushing afterward. That kind of controlled cleaning is very different from operating the membrane year-round in feed water at similar pH.
pH, Bicarbonate, and Carbonate Chemistry: The Scaling Engine
To understand why pH range is so critical, it helps to look briefly at the carbonate system that AXEON and Durpro emphasize.
At lower pH, dissolved carbon exists mostly as carbon dioxide and carbonic acid. As pH rises, more of that carbon converts to bicarbonate ions, and at still higher pH, to carbonate ions. These bicarbonate and carbonate ions interact with calcium and magnesium, the common hardness minerals, to form scaling solids like calcium carbonate.
AXEON recommends design targets that keep total alkalinity in the range of roughly 50 to 150 mg/L and bicarbonate between about 60 and 180 mg/L, while maintaining pH near 6.5 to 7.5. They show that this window stabilizes the carbonate–bicarbonate buffer, keeps LSI near a moderate zone, and supports high salt rejection (often above about 98.5 percent) across common feed types like municipal or brackish water.
Durpro highlights a subtle problem. Carbonate ions contribute relatively little to measured conductivity, especially after RO. That means an RO–deionization system can produce water that appears “good” based on conductivity, while still carrying a significant carbonate load. In deionization stages, anion resins then exhaust faster than expected, increasing chemical consumption and downtime. If you only watch conductivity, you may miss this early. If you also track pH and understand the carbonate speciation, you can spot and manage the real loading.
Taken together, these findings reinforce a practical message. You want the feed pH, alkalinity, and bicarbonate in a zone where: carbonate is low enough to limit scaling; the buffer is stable enough to prevent wild pH swings; and the membrane surface chemistry remains in its optimal state for ion rejection. That is what the 6.5 to 7.5 sweet spot aims to deliver.
Application-Specific pH Targets
Not every RO application has the same tolerance for pH swings or mineral content. AquaComponents summarizes typical feed-water pH targets used in practice for different RO applications.
RO application |
Typical feed-water pH target band |
Rationale |
Brackish water RO |
About 6.0–8.0 |
Balances scaling risk with membrane protection and good rejection. |
Seawater RO |
About 6.0–8.2 |
Slightly narrower upper limit to manage high-salinity scaling. |
Wastewater reuse RO |
About 5.5–8.5 |
Wider band to accommodate variable chemistry while avoiding extremes. |
Boiler feed RO |
About 7.0–8.5 |
Aligned with corrosion and scaling needs of boiler systems. |
Even if you are managing a residential under-sink system rather than a commercial plant, this table is useful. It shows that professionals rarely push membranes anywhere near the extreme pH values on a data sheet in day-to-day operation. In real systems, they keep pH close to neutral and then use specialist cleaning steps at low or high pH only when necessary.
Monitoring and Controlling pH in Real Systems
The good news is that pH is relatively straightforward to monitor and control if you approach it systematically.
For larger or industrial RO systems, AquaComponents and AXEON recommend continuous pH monitoring using online sensors tied into automatic controllers. Acid dosing (commonly using mineral acids such as sulfuric or hydrochloric acid) is used to bring down high-pH feeds and reduce alkalinity. Alkali dosing (often using sodium hydroxide or carbonate-based adjustments) is used to raise overly acidic feeds into the safe operating band. For high-scaling waters, softening or ion exchange pretreatment is often added specifically to remove calcium and magnesium that would otherwise precipitate as carbonate scale at normal operating pH.
Sensorex emphasizes pairing pH monitoring with oxidation–reduction potential (ORP) measurements. ORP tells you how oxidizing or reducing the water environment is, in millivolts. High ORP can indicate active oxidants like chlorine, ozone, or hydrogen peroxide that might attack the membrane, while very low ORP may signal strong reducing conditions or biological activity that raises biofouling risk. Their guidance explains that operators often use ORP readings to tune sodium bisulfite dosing for dechlorination, aiming to keep oxidizers low enough to protect membranes while not overdosing reductants that create their own issues.
Conductivity measurements, both at the RO inlet and outlet, complement pH and ORP by quantifying how much dissolved solids are being removed. Sensorex notes that percent rejection is commonly calculated using inlet and outlet conductivity readings, and that high-purity applications may target roughly 80 to 100 percent rejection. Trends in conductivity, pH, and ORP together paint a much clearer picture of membrane health than any single parameter alone.
In smaller residential systems, you likely will not install full online instrumentation. However, simple digital pH meters or pH test strips, as suggested by homeowner-focused brands like SimPure and Culligan, can quickly confirm whether your feed and product water are within the desired range. When you combine occasional pH checks with routine total dissolved solids measurements, you get a practical snapshot of both water chemistry and membrane performance.
Cleaning, Maintenance, and pH
Even in well-controlled pH ranges, RO membranes gradually foul with biofilm, mineral scale, and fine particles. Multiple sources, including Crystal Quest, AXEON, and DuPont, emphasize that staying ahead of fouling with periodic cleaning and preventive maintenance is much more cost-effective than waiting for severe decline.
Crystal Quest describes two main approaches: removing a residential membrane for soaking, and clean-in-place (CIP) for larger systems. For scale, they recommend low-pH cleaning solutions around pH 2, circulated or soaked for set periods and kept below about 86°F, then followed by flushing with clean, chlorine-free water. For organic fouling or biofilm, high-pH cleaners around pH 12 are used, also within manufacturer-approved temperature and pressure limits.
Industrial-focused DuPont guidance aligns with this. They note that mineral scale can often be removed in roughly six to eight hours of CIP on advanced systems, while some forms of organic or silica-related fouling can require one to two days of carefully managed cleaning. AXEON recommends initiating cleaning when normalized permeate flow declines by about 10 to 15 percent or when differential pressure across the membrane stages rises beyond target values.
For home users, maintenance intervals are simpler but follow the same logic. Affordable Water and Moore Mechanical both stress changing pre-filters every six to twelve months, replacing the RO membrane every two to three years depending on water quality and use, and sanitizing the system annually. These tasks indirectly support proper pH control by removing chlorine before it can degrade the membrane, reducing organic loading that can shift pH behavior, and maintaining consistent flow profiles that help prevent stagnation and localized chemistry extremes inside the system.
The crucial point is this. Cleaning solutions can temporarily operate at extreme pH, but only under controlled conditions and with full respect for the membrane’s cleaning limits. Routine feed pH, on the other hand, should stay within the much narrower range where membranes truly thrive.
Why RO Drinking Water Is Often Slightly Acidic
If you have an RO system at home, you may have measured the pH of the water coming from your faucet and found values between about 5 and 7. Multiple sources, including Culligan, Fliers Quality Water, and iSpring, report this as a common range for RO permeate.
There are two main reasons. First, the RO membrane removes many of the minerals that normally buffer tap water, such as calcium and magnesium. Culligan explains that without these dissolved minerals, the water is less buffered and its pH can change more easily. Second, very pure water readily absorbs carbon dioxide from the surrounding air. iSpring notes that when RO water contacts air, it dissolves CO₂, which forms a weak carbonic acid and nudges pH into the mildly acidic range, often around 5.0 to 5.5 for very pure water, or somewhat closer to 6 to 6.5 depending on conditions.
This slight acidity is not considered a health concern. The Environmental Protection Agency does not treat drinking-water pH as a primary health parameter, and Culligan emphasizes that many everyday beverages such as coffee, tea, and orange juice are significantly more acidic than typical RO water. Fliers Quality Water and SimPure both point out that the body maintains its internal pH tightly within a slightly alkaline range and that normal consumption of RO water in the 5 to 7 range does not significantly shift overall body alkalinity in healthy individuals.
The main practical concern with low pH drinking water is not your body, but your plumbing. Culligan notes that very low pH water can, in rare cases, be mildly corrosive to copper pipes, especially if water sits static in the plumbing for long periods. This is one reason many whole-house systems aim to keep finished water pH within a neutral or slightly alkaline range, often roughly 6.5 to 8.5, consistent with general guideline ranges cited by U.S. authorities.
Adjusting RO Water pH for Taste and Wellness
Even though slightly acidic RO water is safe, many people prefer the taste and mouthfeel of water with a more neutral or mildly alkaline pH and a bit more mineral content. Several manufacturers address this by adding post-treatment stages that raise pH in a controlled way.
Culligan and Nu Aqua describe remineralization or alkaline filters that sit after the RO membrane. These cartridges contain mineral media such as calcium carbonate and other stones that slowly release calcium, magnesium, and sometimes trace elements into the purified water. Nu Aqua notes that such filters can raise pH into a neutral or mildly alkaline range, often somewhere between about 7.0 and 9.5, while also enriching the water with minerals that may improve taste.
Other options, highlighted by Culligan and iSpring, include alkaline mineral drops added directly to pitchers or glasses and mineral-infused storage containers that gradually release minerals into stored RO water. All of these approaches work on the same principle: reintroducing controlled amounts of minerals that both raise pH and restore some buffering capacity.
Claims about systemic health benefits from alkaline water are mixed. Nu Aqua acknowledges that while some studies show benefits such as enhanced calcium uptake and potential support for pH balance, the broader evidence for sweeping health claims remains inconclusive. Fliers Quality Water references research suggesting that alkaline water can serve as a useful calcium source, especially for people who avoid dairy, but presents this as a supportive benefit rather than a medical necessity.
From a Smart Hydration perspective, this suggests a balanced approach. If you enjoy the taste of slightly alkaline water and like the idea of adding some calcium and magnesium back into your RO water, a well-designed remineralization stage can be a practical upgrade. At the same time, you do not need to chase extreme pH values for general health. Keeping pH in a moderate, natural range and focusing on overall water purity and a nutrient-rich diet delivers the most robust and science-backed benefits.
Practical pH Strategies for Protecting Your RO Membrane
If you want to translate all this science into day-to-day action, you can think in terms of a few recurring practices.
First, know the pH and alkalinity of your feed water. A one-time lab test or a professional water assessment will give you a baseline. This matters because a high-alkalinity, high-hardness feed at pH near the upper end of the acceptable band is a scaling event waiting to happen in a high-recovery RO system.
Second, keep your feed pH in the membrane’s “happy zone.” For most residential and light commercial applications, that means targeting something close to neutral, roughly in the 6.5 to 7.5 range, unless your membrane manufacturer gives a more specific recommendation. Avoid long-term operation below about pH 5.5 or above about 8.5 unless your system has been explicitly engineered and chemically conditioned for those conditions.
Third, treat pH control as part of a bigger chemistry strategy. That means pairing pH adjustment with softening, antiscalant dosing, or both when your water has significant hardness or high total dissolved solids. AXEON’s guidelines for antiscalant dosing based on TDS levels illustrate how chemical addition can be scaled to the severity of the water, while continuous pH monitoring keeps the chemistry balanced.
Fourth, integrate pH awareness into your maintenance habits. If your system’s permeate flow drops or salt rejection worsens, check not only filters and pressures, but also pH trends. A shift in feed pH or alkalinity can trigger new scaling or fouling behavior. When you do cleanings, use cleaners that are specifically formulated for RO membranes and stay within the pH, temperature, and pressure limits in your membrane datasheet and cleaner safety information.
Finally, when thinking about drinking water, zoom out from pH alone. Make sure your system is removing health-related contaminants such as lead, PFAS, and arsenic, as highlighted by brands like Culligan and Fliers Quality Water. Use remineralization or mild alkalization primarily to fine-tune taste and support comfortable hydration, rather than chasing extreme pH numbers.
Short FAQ
Is slightly acidic RO water safe to drink every day?
Yes, the research and manufacturer guidance summarized by Culligan, Fliers Quality Water, and SimPure indicates that RO water with pH in the 5 to 7 range is generally safe for everyday drinking for most healthy people. The body tightly regulates its own internal pH, and common beverages like coffee and orange juice are significantly more acidic than typical RO water.
Can low or high pH damage my RO membrane?
Persistent low pH can promote hydrolysis and chemical attack on membrane materials, while persistent high pH accelerates scaling and can drive irreversible fouling, especially above about pH 10.5. Sources such as BestMembrane, AquaComponents, AXEON, and Durpro all warn that while membranes can survive short cleaning cycles at extreme pH, they perform best and last longest when feed pH is kept near neutral.
Do I really need an alkaline filter on my home RO system?
You do not need an alkaline filter for safety. Slightly acidic RO water in the typical 5 to 7 range is considered safe to drink if your overall diet is balanced. Alkaline filters and remineralization stages are mainly about taste and personal preference, with a secondary benefit of gently raising pH and adding back minerals like calcium and magnesium. As Nu Aqua notes, broad health claims about alkaline water remain scientifically uncertain, so it is best to view these filters as comfort and taste upgrades rather than medical treatments.
When you keep pH in the right range, you give your RO membrane the environment it was designed for: stable surface chemistry, manageable scaling, strong contaminant rejection, and long service life. Whether you are tuning a high-recovery system for a facility or dialing in your under-sink filter at home, thoughtful pH control turns your RO from “just a filter” into a dependable hydration partner that quietly protects both your health and your equipment.
References
- https://mooremech.net/reverse-osmosis-system-maintenance-tips/
- https://www.affordablewaterinc.com/reverse-osmosis-maintenance-tips-for-first-time-owners
- https://bestamembrane.com/factors-affecting-ro-membrane-filtration-systems/
- https://www.dupont.com/knowledge/importance-of-industrial-ro-system-maintenance.html
- https://espwaterproducts.com/pages/reverse-osmosis-maintenance?srsltid=AfmBOorwSqrps918vAsDEU04cH3b3PZDY3hs0LeuH8Mv5o_gshpKw_8p
- https://fliersqualitywater.com/the-ph-of-reverse-osmosis-water/
- https://www.ispringfilter.com/ac/why-reverse-osmosis-water-is-acidic?srsltid=AfmBOopIEHKUV9E374Vq541kdhDgsMO4GkGPWTK9plDG4PiH94NRevJZ
- https://sensorex.com/orp-ro-systems/?srsltid=AfmBOopwJ1yg68uDArfWqKASpgnkRehcivBssukgE9Gy_Bfal5pWPfqo
- https://www.theperfectwater.com/faq/what-is-ph-and-why-is-it-important.html?srsltid=AfmBOop8iiDu0-1VW5780wa0ofctQRs-EXzMu5zrySu5fCFE4pUtqLsR
- https://www.axeonwater.com/blog/best-practices-for-operating-and-maintaining-reverse-osmosis-systems/

Share:
How Long Do Silver Ion Coatings Really Last on Antibacterial Water Tanks?
Understanding When and Why to Boost RO Pressure Toward 0.6 MPa (About 87 PSI)